Baeyer's Test for Alkanes
Web The Baeyer test yielded a oily brown precipitate. I could then expect a similar result when the product was tested for unsaturation using the Baeyer test.

Tests For Unsaturation Chemistry Practicals Class 12
Bromine water is an orange.

. The two control tests had results as expected. When potassium permanganate reacts with an alkene the solution changes from a purple color to a brown. The Baeyer test uses dilute Pottasium Permanganate to Oxidize the carbon-carbon double or triple bond.
The baeyers test is used to test for an unsaturated carbon carbon bond such as an alkene or alkyne but not an aromatic carbon carbon bond. Bromine water is an orange solution of bromine. The Baeyer test for unsaturation is for determining the presence of carbon-carbon double bonded compounds called alkenes or carbon-carbon trible bonded compounds called alkyne bonds.
When it is added to alkanes the purple color does not change. Web In fact most alkanes are so unreactive you will observe this in todays experiment that the only reaction that they undergo is combustion combining with molecular oxygen to produce carbon dioxide and water. Web Baeyers Test.
C baeyer test alkane c 7 h 16 kmno 4 no reaction no. Web There are two methods for detecting the unsaturation in an organic compound. The activity can be extended to include testing the samples with a 1 potassium manganate vii solution.
Reaction with Aqueous Potassium Permanganate. The test for unsaturation is demonstrated. Web Baeyers test Oxidation with alkaline solution of KMnO 4.
Alkaline solution of potassium permanganate is known as Baeyers reagent. Course Title BAHASA MELAYU 0187701722. Dissolve the organic compound in 2ml of water or acetone in the test tube.
Potassium permanganate is an oxidizing agent that reacts with unsaturated aliphatic hydrocarbons but does not react with alkanes or aromatic hydrocarbons. Web You can get all kinds of articles on baeyers test for alkanes here. Web C Baeyer test Alkane C 7 H 16 KMnO 4 no reaction No reaction occur Alkene C 6 H.
Web Gokarna ChemistryBaeyers reagent test of alkene unsaturation test of alkeneFor more videos on chemistry please likesubscribe and commentGokarna Chemistry. Observe the solution if pink colour persists then it is saturated compound. Small samples of the liquids are also ignited and the appearance of the flames compared.
The dilute KMnO 4 solution has a deep purple color. Alkaline potassium permanganate test Baeyers test. Potassium permanganate does not react with alkanes because they are saturated single bonds which are all taken.
Web The Baeyer reagent is a cold dilute aqueous solution of potassium permanganate which is a deep purple color. If there is no reaction you should see no color change. It becomes colourless when it is shaken with an alkene.
Web The presence of the CC double bond allows alkenes to react in ways that alkanes cannot. This allows us to tell alkenes apart from alkanes using a simple chemical test. Web This allows us to tell alkenes apart from alkanes using a simple chemical test.
Web Alkynes and baeyers reagent. The Baeyer test for unsaturated hydrocarbons involves reaction with a hydrocarbon with alkene or alkyne like double bonds. Alkanes alkenes and alkynes are prepared by electrolysis of salt of monocarboxylic acid dicarboxylic acid and unsaturated dicarboxylic acid respectively.
Alkyne compounds can be oxidized to vicinal diketones or dialdehyde Vic-diketones or 12-diketones or under more vigorous conditions carboxylic acids by baeyers reagent. Potassium permanganate KMnO4 solution is a purple color. Web This activity compares the reaction with bromine water of several liquid alkanes and alkenes.
The Baeyer test for unsaturation is for determining the presence of carbon-carbon double bonded compounds. Take a little of the given organic compound to be tested in a test tube. Alkaline potassium permanganate test Baeyers test Bromine test.
In this test the pink colour potassium permanganate disappears when an alkaline potassium permanganate is added to an unsaturated. It was good to test the controls because of the unique nature of the Baeyer test on the cyclohexene which was an oily brown precipitate. Add 1 alkaline potassium permanganate solution dropwise and shake the mixture.
School Sekolah Menengah Kebangsaan Kota Masai 2. Web Test for alkenes Baeyers Reagent test Bromine water test Ozonolysis of alkanes testABOUT THE VIDEO -1 Alkenes introduction2 Bayers reagent test f. More vigorous conditions can be made by increasing the alkalinity of the solution by adding potassium hydroxide KOH.
Its called oxidation because the double.

Baeyer S Reagent Definition Preparation And Reaction

Baeyer S Reagent Definition Preparation And Reaction

Baeyer And Bromine Test For Unsaturation Presence Of Alkenes And Alkynes Youtube
What Is Bayer S Test What Is Its Mechanism Quora

Baeyer S Test And Bromine Water Test For Detection Of Carbon Carbon Double Bond Youtube
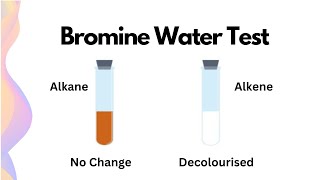
Bromine Water Test Identifying C C Bonds Hsc Chemistry Youtube
Comments
Post a Comment